Almost 100 years after it was first commercialized for use in refrigeration and air conditioning units, and several decades after global production was essentially eliminated, CFC-12 (CF2Cl2, dichlorodifluoromethane, chlorofluorocarbon-12) still makes a considerable contribution to climate warming. But how bad for climate are halocarbons and their numerous replacement gases? A recent article in Reviews of Geophysics compares the greenhouse gas strength for hundreds of halocarbons and related compounds and quantifies the contribution of these gases to the radiative forcing of climate change. Here, some of the authors provide an update on the history and importance of halocarbons for climate and the environment.
What kind of gases are halocarbons and where do they originate?
Halocarbons comprise a wide range of gases. They are compounds that only contain carbon and one or more halogens such as fluorine, chlorine and bromine. Halocarbons are mostly man-made chemicals and have been used in a range of applications over the past century including as solvents, fire-fighting agents, and refrigerants.
Prior to the 1930s, refrigerators and air conditioning systems were used mainly by industry and used noxious and toxic gases such as NH3, CH3Cl, and SO2 as refrigerants. However, it was recognized that safer refrigerants were needed for widespread household usage.
Thomas Midgley working at Frigidaire Corporation, a subsidiary of General Motors, developed CFC-12 as a refrigerant that was not only non-toxic and non-flammable but also odorless, tasteless, colorless, chemically inert, and inexpensive!

CFC-12 was rapidly adopted by the refrigeration and air conditioning industries and found many other uses such as an aerosol propellant and foam blowing agent.
Global production of CFC-12 increased exponentially to over 400 kt/year by the early 1970s.
Unfortunately, we were not aware of the environmental problems related to emissions of CFC-12 and other chlorofluorocarbon (CFC) gases until the mid-1970s.
The environmental impact was recognized in the United Nations Montreal Protocol on Substances that Deplete the Ozone Layer (1987) and, as a result, global production was essentially eliminated by the 1990s. However, CFC-12 is still the most abundant halocarbon in the atmosphere.
What impact do halocarbons have on the climate?
The most widely known concern related to the emissions of halocarbons is their ability to destroy stratospheric ozone [Molina and Rowland, 1974].
The ozone layer shields us against dangerous ultraviolet radiation from the sun, and ozone depletion could lead to harmful effects such as increased risk of skin cancer. It is also a cause of climate change.
The discovery made in the 1970s, that chlorine- and bromine-containing halocarbons cause ozone depletion, led to the 1995 Nobel Prize in Chemistry being awarded to Paul Crutzen, Mario Molina, and Sherwood Rowland.
However, another major concern about emissions of halocarbons is that many of them are powerful greenhouse gases. Several of them stay in the atmosphere for decades and are thousands of times more efficient (per kg emitted) at warming the Earth than CO2, although emissions of CO2 as a result of human activity, are much higher than the halocarbons.
Although CFCs have mostly been replaced by gases that have low or no potential for ozone depletion, such as hydrochlorofluorocarbons (HCFCs) or hydrofluorocarbons (HFCs), many of the replacement gases are also strong greenhouse gases.
Is it possible to quantify the contribution of halocarbons to global warming?
The impact of a greenhouse gas on the Earth’s climate system is typically quantified in terms of its radiative forcing, which is the net change in the energy balance of the Earth caused by the change in concentration of a gas in the atmosphere.
When accounting for the historical change in the atmospheric abundance of the different gases, we find that the present-day radiative forcing of halocarbons and related compounds is about 18% of the current CO2 forcing.
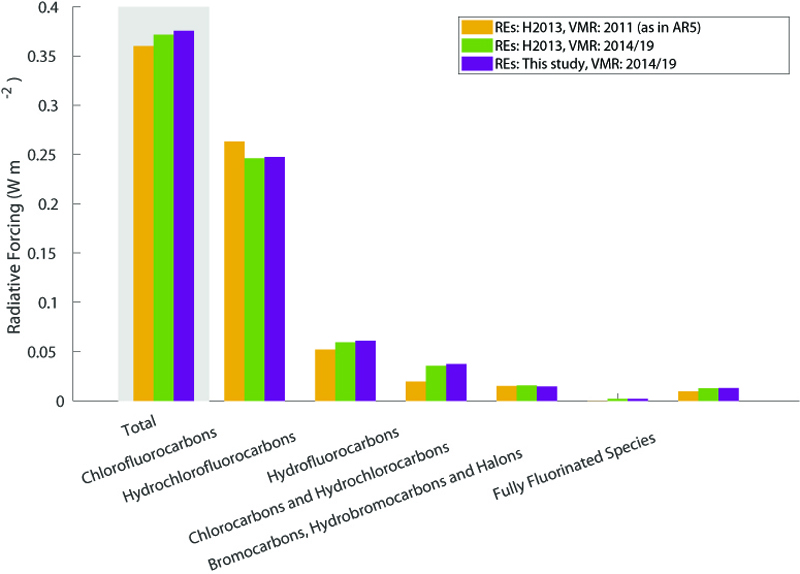
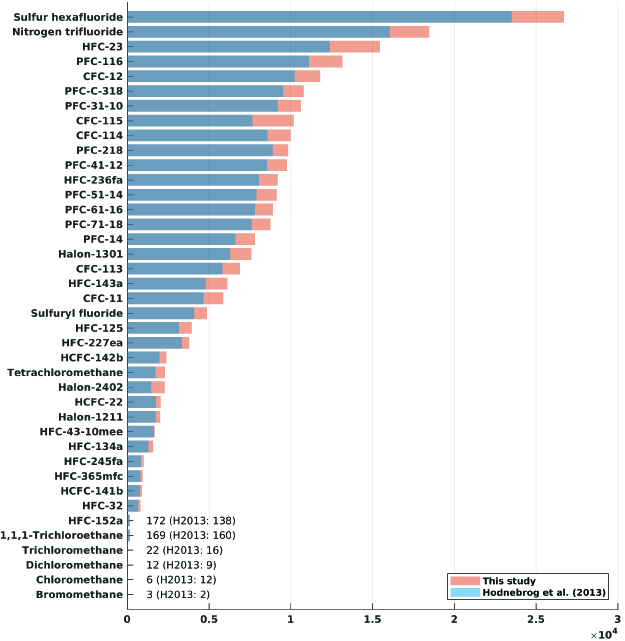
The most commonly used metric in policy regulations, although the subject of debate, is the global warming potential (GWP) for a 100-year time horizon. GWP values have been provided, and regularly updated, in all assessment reports by the IPCC since their first report in 1990 and in scientific assessment reports on ozone depletion by the World Meteorological Organization.
As an example, our study shows a GWP 100-year value for CFC-12 of nearly 12,000, meaning that with this metric, CFC-12 has a climate impact nearly 12,000 times greater than CO2 per kg emitted.
What changes could policy makers implement to reduce the impact of halocarbons on climate?
First, it should be said that much has already been done. The Montreal Protocol has been a major success as it led to the phase-out of CFCs and other ozone-depleting gases. As a consequence, the ozone layer has slowly started to heal [Solomon et al., 2016]. Interestingly, the Montreal Protocol has had a greater climate benefit than the Kyoto Protocol which was designed to reduce global warming [Velders et al., 2007].
One of the main challenges now is the rising concentrations of some replacement compounds with high GWP values, such as some HFCs. The 2016 Kigali Amendment to the Montreal Protocol includes controls on emissions of HFCs, but given the large number of gases and that new compounds are constantly being developed, policy makers must ensure that the atmospheric concentrations of important gases are closely monitored and that efficient measures are in place to limit emissions of strong greenhouse gases. A good observational network is important to detect and understand any unexpected rise in emissions of banned compounds, such as the recent CFC-11 emissions from China [Rigby et al., 2019], which could lead to additional warming and a delay of the ozone layer recovery.
It is also important to stress that these gases only impact climate if they leak from the system they are used in (e.g. refrigeration units and air conditioning units in cars and buildings), either while in use or when they are decommissioned. So, policymakers and manufacturers can work together to ensure that leakage is minimized and the gases are recovered at the end of a product’s lifetime.
What are some of the unresolved questions where additional research, data or modeling is needed?
One need for additional research is to better understand how halocarbons influence different parts of the climate system. A new definition of radiative forcing called “effective radiative forcing” (ERF) includes so-called rapid adjustments, which involve fast changes in e.g. atmospheric temperature, water vapor and clouds.
Quantifying these processes, and thereby the ERF, leads to a better indication of how the gas impacts surface temperature. A challenge is that ERF requires computationally expensive calculations for each gas using global climate models, but similar quantifications have recently been made for other major climate drivers such as CO2 and methane [Smith et al., 2018].
Another challenge is that laboratory measurements of the absorption features of a gas, which is key input to radiative forcing calculations, are lacking for many gases. While computational methods for determining absorption features exist, and are sometimes provided for hundreds of gases (e.g., Papanastasiou et al., 2018), these have much greater uncertainty than traditional laboratory measurements. Research is needed to improve the accuracy of computational methods and expand and refine the laboratory measurement database.
—Øivind Hodnebrog (oivind.hodnebrog@cicero.oslo.no; ![]() 0000-0001-5233-8992), Center for International Climate Research, Norway; Keith P. Shine (
0000-0001-5233-8992), Center for International Climate Research, Norway; Keith P. Shine (![]() 0000-0003-2672-9978), Department of Meteorology, University of Reading, UK; and Timothy J. Wallington (
0000-0003-2672-9978), Department of Meteorology, University of Reading, UK; and Timothy J. Wallington (![]() 0000-0002-9810-6326), Research & Advanced Engineering, Ford Motor Company, USA
0000-0002-9810-6326), Research & Advanced Engineering, Ford Motor Company, USA
